Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Metal Ion Uptake Properties of Chelating Ion-Exchange Copolymer Synthesized from 2, 4- Dihydroxypropiophenone and 4-Pyridylamine
Authors: N. C. Das, W. B. Gurnule
DOI Link: https://doi.org/10.22214/ijraset.2024.63689
Certificate: View Certificate
Abstract
The chelating ion exchange copolymer 2,4-DHP-4-PAF-II has been synthesized by condensing 2,4-dihydroxypropiophenone, 4-pyridylamine and formaldehyde in the presence of 2M hydrochloric acid as catalyst using 2:1:3 molar proportion of reacting monomers. The resulting resin has been characterized by elemental analysis, UV-Visible, FT-IR, and 1H-NMR. The morphological feature of copolymer has been studied by scanning electron microscopy (SEM) .The chelation cation-exchange properties of the copolymer resin has been studied for Co2+, Ni2+, Cu2+, Zn2+ and Pb2+ ions. In the study of the selectivity of metal-ion uptake involving the measurements of the distribution of a given metal ion between the copolymer sample and a solution containing the metal ion, a batch equilibrium method was used. Copolymer showed a higher uptake capacity for Ni2+ and Cu2+ ion than Zn2+, Co2+, and Pb2+ ions.
Introduction
I. INTRODUCTION
The effluents from mines and metal industries containing heavy metal ions are increasingly discharged into the environment. Unlike some organic pollutants, heavy metal ions are not biodegradable and cannot be metabolized or decomposed. They are responsible for causing damages to the environment and can also easily enter the food chain through a number of pathways and adversely affecting the health of people. The traditional methods commonly used for their removal from aqueous solution include ion-exchange, solvent extraction, chemical precipitation, nano-filtration and reverse osmosis. Now a day, among the various solid adsorbent, polymeric chelating resins are widely used in the removal of heavy metal ions due to their high adsorption capacities and selectivity [1].
Separation of toxic metal ions from waste water using pyrogallol-biuret-formaldehyde copolymer resin has reported by Rahangdale et al. [2]. Gurnule and coworker prepared chelating ion-exchange resin from phallic acid - melamine - formaldehyde and characterized by elemental analysis, FT-IR and 1H-NMR spectra. Metal ion uptake capacity of synthesized copolymer has been carried out by Batch equilibration method for different metal ions at different concentrations [3]. Comparative study of strong anion exchange poly (Styrene-co-EGDMA-co-VBC) and strong anion exchange hypercrosslinked poly (HEMA-co-EGDMA-co-VBC) was carried out by N. Abdullah et al [4].
Dhote et al. synthesized a ion exchange resin from 4-hydroxybenzophenone and melamine with formaldehyde which was characterized and chelating nature of prepared resin was studied with Cd (II), Ni (II), Zn (II), Cu (II), and Pb (II) metal ions [5]. Gurnule et al. have studied the chelation ion exchange properties of copolymer resin synthesized from 1,5-diaminonaphthalene, 2,4-dihydroxypropiophenone and formaldehyde [6].
Masram et al. have been synthesized the copolymer resin from salicylic acid and diaminobenzoic acid with formaldehyde and studied the chelating ion-exchange properties [7]. Nandekar et al. synthesized a copolymer resin by condensation of salicylic acid, semicarbazide and formaldehyde and studied its ion-exchange properties for Zn (II), Cu (II), Co (II), Ni (II), and Pb (II) ions [8]. Synthesis, antimicrobial and ion-exchange studies of copolymer resin derived from substituted resorcinol, biuret and formaldehyde have been studied by Ravichander et al. [9].
The present research deals with synthesis, characterization of copolymer 2,4-DHP-4-PAF-II prepared by condensing 4-pyridylamine, 2,4-dihydroxypropiophenone, and formaldehyde and reported for its metal ion uptake capacity for Cu2+, Ni2+, Co2+, Zn2+ , and Pb2+ ions.
II. MATERIALS AND METHODS
The important chemicals like 4-pyridylamine, 2, 4-dihydroxypropiophenone, formaldehyde, DMF, DMSO, THF, acetone and diethylether were procured from the market and were of chemically pure grade.
A. Synthesis of 2, 4-DHP-4-PAF-II copolymer
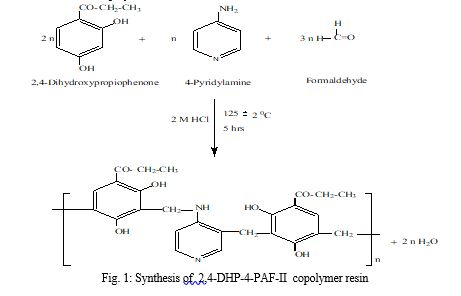
B. Physico-chemical and Analytical Studies
The copolymer resin was subjected to elemental analysis for carbon, hydrogen and nitrogen on Elemental Vario EL III Carlo Erba 1108. Infrared spectra was carried out in the range of 4000-500 cm-1 in KBr pellets in najol mull on Perkin-Elmer-Spectrum RX-I, FT-IR Spectrophotometer. The 1H-NMR spectrum of copolymer was scanned on Bruker Advance -II 400 MHz NMR spectrophotometer using DMSO-d6 as a solvent. All the spectral studies were carried out at STIC, Cochin University, Cochin, India.
C. Ion-exchange Properties
The metal ion uptake capacity of copolymer 2,4-DHP-4-PAF-II as an ion exchanger was determined by Batch equilibrium method. We studied the influence of a variety of electrolytes at different concentration and pH, the rate of metal ion uptake and distribution of metal ion between the solution and copolymer phase.
D. Estimation of metal ion uptake in the presence of electrolytes of different concentrations
Copolymer sample 25 mg was suspended in 25 ml NaNO3 electrolyte solution of known concentration. The pH of the solution was adjusted by using either 0.1N NaOH or 0.1N HCl. The suspension was stirred at temperature 25 0C for of 24 hrs. To this suspension 2 ml of 0.1M solution of the metal ion was added and the required pH was adjusted. The mixture was again stirred for 24 hr at 25 0C and filtered. The copolymer was then filtered off and washed with distilled water. The filtrate and washings were collected and then the metal ion content was estimated by titration against standard EDTA. Same titration was carried out without copolymer sample (blank reading). The amount of metal ion uptake of the polymer was calculated from the difference between the reading in actual experiments and a blank experiment without polymer. The experiment was repeated in the presence of several electrolytes such as NaCl and NaClO4, Na2SO4 at different concentrations. The metal ion uptake can be determined as
Metal ion adsorbed (uptake) by resin = (X-Y) Z mmol/g
Where Z (ml) is the difference between actual experimental reading and blank reading; X (mg) is a metal ion in 2 ml 0.1 M metal nitrate solution before uptake; Y (mg) is a metal ion in 2 ml 0.1 M metal nitrate solution after uptake.
By using this equation, the uptake of various metal ions by the resin can be calculated and expressed in term of millimols per gram of copolymer [10].
E. Estimation Of The Rate Of Metal Ion Uptake As A Function Of Time
In order to estimate the time required to reach the state of equilibrium under given experimental conditions, a series of experiments of the type described above were carried out, in which the metal ion uptake by chelating resins was estimated from time to time in the presence of 25 ml of 1M NaNO3 solution at 25 0C. It was assumed that under given conditions, the state of equilibrium was established within 24 hrs. Rate of metal ions uptake is expressed as percentage of the amount of metal ions taken up after a certain time.
Metal ion uptake at different time (%) = Metal ion adsorbed/ Metal ion adsorbed at equilibrium × 100
The percentage amount of metal ion taken up at different time is defined as: Percentage of metal ion adsorbed after 1 h = (100X)/Y
Where, X is mg of metal ion adsorbed after 1h and Y is mg of metal ion adsorbed after 24h. Then, by using this expression, the amount of metal adsorbed by copolymer after specific time intervals was calculated and expressed in term of percentage metal ion adsorbed [11].
F. Evaluation of the distribution of metal ions at different pH
The distribution of each of the five metal ions Cu(II), Co(II), Ni(II), Zn(II) and Pb(II) between the copolymer phase and aqueous phase was evaluated at 25 0C in the presence of 1M NaNO3 solution. As described earlier, the experiments were carried out at different pH values. The Distribution ratio (D) was estimated with the following relationship
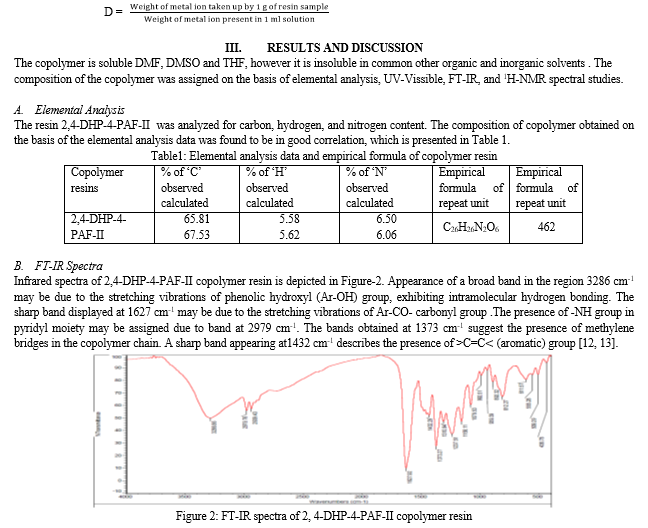
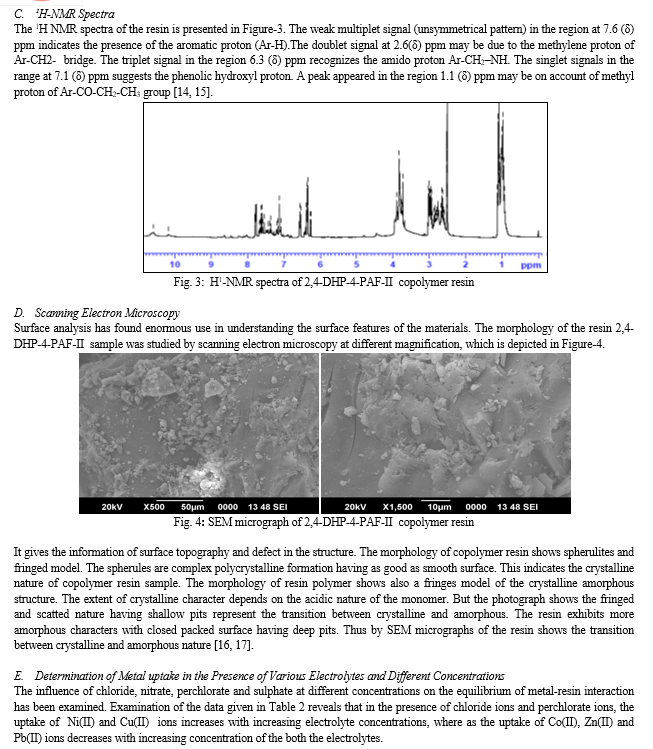
Table 2: Evaluation of the effect of different electrolytes on the uptakea of several metal ions by 2,4-DHP-4-PAF-II copolymer
|
Metal ion |
pH |
Conc. |
Weight of metal ion (in mg.) taken up in the presence of different electrolyte |
|||
|
NaClO4 |
NaCl |
NaNO3 |
Na2SO4 |
|||
|
|
|
0.01 |
2.25 |
2.47 |
1.70 |
3.66 |
|
|
|
0.05 |
2.56 |
2.71 |
1.96 |
2.51 |
|
Cu2+ |
4.5 |
0.10 |
2.94 |
2.94 |
2.67 |
2.30 |
|
|
|
0.50 |
3.26 |
3.53 |
3.14 |
1.47 |
|
|
|
1.00 |
3.44 |
3.84 |
3.48 |
0.87 |
|
|
|
0.01 |
1.91 |
1.26 |
1.30 |
2.97 |
|
|
|
0.05 |
2.66 |
1.34 |
1.81 |
2.26 |
|
Ni2+ |
4.5 |
0.10 |
3.07 |
1.46 |
2.14 |
1.69 |
|
|
|
0.50 |
3.58 |
1.85 |
2.34 |
0.91 |
|
|
|
1.00 |
4.14 |
2.29 |
2.61 |
0.70 |
|
|
|
0.01 |
1.60 |
1.66 |
1.81 |
1.76 |
|
|
|
0.05 |
1.39 |
1.35 |
1.55 |
1.58 |
|
Co2+ |
5.0 |
0.10 |
1.23 |
1.19 |
1.30 |
1.37 |
|
|
|
0.50 |
0.87 |
0.91 |
0.99 |
1.15 |
|
|
|
1.00 |
0.53 |
0.70 |
0.70 |
0.99 |
|
|
|
0.01 |
1.76 |
1.59 |
2.38 |
1.96 |
|
|
|
0.05 |
1.45 |
1.25 |
2.14 |
1.64 |
|
Zn2+ |
5.0 |
0.10 |
1.23 |
1.06 |
1.71 |
1.35 |
|
|
|
0.50 |
0.94 |
0.91 |
1.20 |
1.14 |
|
|
|
1.00 |
0.67 |
0.44 |
0.65 |
0.89 |
|
|
|
0.01 |
1.50 |
1.80 |
1.84 |
2.14 |
|
|
|
0.05 |
1.31 |
1.47 |
1.63 |
1.86 |
|
Pb2+ |
6.0 |
0.10 |
1.16 |
1.29 |
1.44 |
1.39 |
|
|
|
0.50 |
0.99 |
0.96 |
1.14 |
0.99 |
|
|
|
1.00 |
0.76 |
0.55 |
0.73 |
0.71 |
a[M(NO3)2] = 0.1 mol/l; Volume = 2 ml; Volume of electrolyte solution : 25 ml
Weight of resin = 25 mg; Time: 24 h; Room temperature
But in the presence of nitrate ions, the uptake of Ni(II) and Cu(II) ions increases with increasing concentration of the electrolyte where as the uptake of Zn(II), Co(II), and Pb(II) ions decreases with increasing concentration of NaNO3 electrolyte. However, in the presence of SO42- ions, the uptake of Ni(II), Cu(II), Co(II), Zn(II), and Pb(II) ions decreases with increasing concentration of Na2SO4 electrolyte. This is due to perchlorate ions, chloride ions and nitrate ions forms weak complexes with Cu (II) and Ni(II) ion while sulphate forms strong complexes with Cu(II) and Ni(II). But perchlorate, chloride and nitrate ions forms strong complexes with Co(II), Zn(II) and Pb(II) [18, 19].
F. Rate of Metal ion uptake as a Function of Time
To find out the time required to reach the equilibrium, the rates of metal ion absorption by 2,4-DHP-4-PAF-II resin samples were estimated for Ni(II), Co(II), Cu(II), Zn(II) and Pb(II), ions. The dependence of the rate of metal-ion uptake on the nature of the metal ions which is shown in Table-3. These results indicate that under the given conditions, the time taken for the uptake of the different metal ion depends on the nature of the metal ions.
A careful analysis of the estimated data, reveals that to attain the equilibrium, Cu(II), Ni(II), Zn(II) and Co(II) ions required about 5 hrs where as Pb(II) ions required almost 6 hrs. The experimental results shows that the rate of metal-ion uptake of Cu(II), Ni(II), Co(II), Zn(II) is more than Pb(II). This trends is due to Cu(II), Ni(II), Co(II), Zn(II) have nearly equal ionic size but Pb(II) has comparatively large ionic size [20-22].
Table 3: Comparison of the rates of metal (M) ion uptakea by 2,4-DHP-4-PAF-II copolymer
|
Metal ion
|
pH |
% of metal ion uptakeb at different time (hrs.) |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
||
|
Cu2+ |
4.5 |
56.6 |
68.7 |
81.3 |
88.4 |
93.2 |
- |
|
Ni2+ |
4.5 |
61.7 |
70.1 |
80.5 |
85.1 |
94.2 |
- |
|
Co2+ |
5 |
46.3 |
65.2 |
76.3 |
85.4 |
94.6 |
- |
|
Zn2+ |
5 |
49.1 |
60.1 |
71.1 |
88.2 |
92.1 |
- |
|
Pb2+ |
6 |
27.2 |
48.3 |
63.4 |
74.1 |
83.3 |
94.1 |
a [M(NO3)2]= 0.1 mol/l; volume : 2ml; NaNO3 = 1.0 mol/l; volume: 25ml, Room temperature.
b Metal ion uptake = (Amount of metal ion absorbed x 100) / amount of metal ion absorbed at equilibrium.
G. Distribution Ratios of Metal ions at Different pH
The effect of pH on the amount of metal ions distributed between two phases can be explained by the result shown in the Table 4. The data on the distribution ratio as a function of pH, indicates that the relative amount of metal ions uptake by the 2,4-DHP-4-PAF-II copolymer increases with increasing pH of the medium. To prevent hydrolysis of the metal ions at higher pH, the study was carried out up to a definite pH value for the particular metal ion. However, the increase magnitude is different for different metal ions. The distribution of Cu(II) and Ni(II) metal ions is more as compare any other metal ions under investigation.
Table 4: Distribution Ratio ‘D’a of Different Metal Ionsb as a function of different pH of 2,4-DHP-4-PAF-II Copolymer resin
|
Metal ions |
Distribution ratios of different metal ions at different pH |
|||||||
|
1.5 |
2.0 |
2.5 |
3.0 |
3.5 |
4 |
5 |
6 |
|
|
Cu2+ |
- |
- |
64.7 |
88.4 |
193.2 |
344.3 |
771.1 |
1048.2 |
|
Ni2+ |
- |
- |
57.1 |
66.2 |
92.2 |
237.1 |
452.1 |
741.2 |
|
Co2+ |
- |
- |
40.1 |
55.3 |
88.2 |
128.1 |
264.4 |
455.2 |
|
Zn2+ |
- |
- |
44.1 |
51.5 |
80.2 |
95.3 |
180.3 |
252.1 |
|
Pb2+ |
- |
- |
41.2 |
61.3 |
83.3 |
112.3 |
156.3 |
255.7 |
aD = weight (in mg) of metal ions taken up by 1g of copolymer/weight (in mg) of metal ions present in 1ml of solution.
b [M(NO3)2]= 0.1 mol/l; volume : 2ml; NaNO3 = 1.0 mol/l; volume: 25ml, time 24h (equilibrium state) at Room temperature.
The order of distribution ratio of metal ions is found to be Cu(II) > Ni(II) > Co(II) > Zn(II) > Pb(II). The results of such type of study are helpful in selecting the optimum pH for a selective uptake of a particular metal cations from a mixture of different metal ions [23, 24].
IV. ACKNOWLEDGEMENT
The authors are thankful to the Principal, Kamla Nehru Mahavidyalaya, Nagpur for providing necessary laboratory facilities and also pleased to express their gratitude to the Director, STIC, Cochin University, Cochin, for carrying out spectral studies.
Conclusion
The 2,4-DHP-4-PAF-II copolymer was prepared from 2, 4-dihydroxypropiophenone and 4-pyridylamine with formaldehyde in the presence of acid by condensation techniques. The structure of the copolymer was confirmed by physico-chemical, UV-Vissible, FT-IR, and 1H-NMR spectral studies. The semi crystalline nature of the copolymer was confirmed by the SEM analysis and reveals that resin can act as an ion exchanger for various metal ions such Ni2+, Cu2+ , Co2+, Zn2+ and Pb2+ ions. Since the copolymer contains phenolic (-OH) group and amino (NH2 ), it play a key role in the ion exchange phenomenon, due to its higher tendency of capturing metal ions. Metal uptake capacity of the resin shows a higher selectivity for Ni2+ and Cu2+ ion than for Co2+, Zn2+ and Pb2+ ions.
References
[1] A. Masoumi, M. Ghaemy, Adsorption of heavy metal ions and azo dyes by cross linked nano chelating resin, Express Polymer Letters, 8(3), (2014), 187-196. [2] S. S. Rahangdale, Dinesh D. Kamdi, Jyotsna V. Khobragade and Wasudeo B. Gurnule, Separation of toxic metals ions from waste water using pyrogallol – biuret - formaldehyde copolymer resin, International Journal of Researches in Biosciences, Agriculture and Technology, 8(3), (2022), 259-268. [3] W. B. Gurnule and J. V. Khobragade, Removal of heavy metals using ion-binding copolymer involving pthalic acid with formaldehyde by batch equilibrium technique, I J R B A T, 5(2), (2017), 1-9. [4] N. Abdullah, M. Z. Yahya and R. Mahmod, Comparative study of strong anion exchange hypercrosslinked poly (HEMA-co- EGDMA-co-VBC) and strong anion cxchange poly(styrene-co-EGDMA-co-VBC): Synthesis and characterization, Chem. Engg. Res. Bull., 19, (2017), 96-101. [5] S. P. Dhote, W. B. Gurnule and A. B. Zade, Metal ion binding properties of a terpolymer resin: synthesis, and its application as a ion exchanger, IOSR, J. Appl. Chem., ISSN: 2278-5736, (2014), 35-39. [6] W. B. Gurnule, Y. U. Rathod, V. U. Pandit, N. C. Das, Chelation ion exchange properties of copolymer resin synthesized from 1,5-diaminonaphthalene, 2,4-dihydroxypropiophenone and formaldehyde, Materials Today:Procedings, 53, (2022), 80-85. [7] D. T. Masram, K. P. Kariya and N. S. Bhave, Synthesis of resin-VI: Salicylic acid, diaminobenzoic acid and formaldehyde resin and its ion exchange properties, Der Pharma Chemica, 6(3), (2014), 292-299. [8] K. Nandekar, S. Mandavgade and W. B. Gurnule, Chelation ion-exchange properties of copolymer resin derived from salicylic acid, semicarbazide and formaldehyde, J. Inform. Knowledge, Res. Human. Soc., 1(4), (2016), 177-188. [9] M. Ravichander, G. A. Reddy, V. Malathi, J. Pochampalli and R. Thampu, synthesis, antimicrobial and ion exchange studies of some terpolymer resins derived from substituted resorcinol, biuret and formaldehyde, J. Chem. Pharm. Res., 7(9), (2015), 868-871. [10] S. S. Katkamwar, M. Ahmed and W. B. Gurnule, Studies on chelation ion-exchange properties of copolymer resin derived from p-cresol,dithio oxamide and formaldehyde, J. Environ. Res. Develop., 1(7), (2012), 330-337. [11] R. N. Singru, Semiconducting and chelating applications of newly synthesized terpolymer, Advances in Applied Science Research, 2(6), (2011), 206-214. [12] W. B. Gurnule and N. C. Das, Electrical conducting behavior of copolymer resin-III synthesized from 2,4-dihydroxypropiophenone, 4-pyridylamine and formaldehyde, Ajanta, 8(1), (2019), 16-25. [13] W. B. Gurnule, Jyotsana Khobragade and Mudrika Ahamed, Thermal degradation studies of high performance copolymer resin derived from 8-hydroxyquinoline 5-sulphonic acid, semicarbazide and formaldehyde, Der Pharma Chemica, 6(5), (2014), 334-342. [14] M. M. Yeole, S. Shrivastava and W. B. Gurnule, Synthesis and characterization of copolymer resin derived from 4-methylacetophenone, phenylhydrazine and formaldehyde, Der Pharm. Chem., 7(5), (2015), 124-129. [15] Kamlakar. A. Nandekara, Jivan. R. Dontulwara and Wasudeo. B. Gurnule, Thermal Behaviour of Newly Synthesized Copolymer Derived from Salicylic acid, and Thiosemicarbazide, Der Pharma Chemica, 4(4), (2012), 1644-1652. [16] N. C. Das and W. B. Gurnule, Synthesis, characterization and morphology of organic copolymer resin-III resulting from 2,4- Dihydroxypropiophenone, 1,5-diaminonaphthalene and formaldehyde, IJARSCT, 4(12), (2021), 114-121. [17] W. B. Gurnule, J. Khobragade and M. Ahamed, Synthesis, characterization and chelation ion exchange properties of copolymer resin , Der Pharmacia Lettre, 6 (6), (2014), 297-303. [18] N. C. Das, W. B. Gurnule, Y. U. Rathod and V.U. Pandit, Studies of chelation ion exchange properties of copolymer resin derived from 2,4- Dihydroxypropiophenone, 1,5-diaminonaphthalene and formaldehyde, Materials Today: Proceedings, 53, (2022), 80-85. [19] K. Nandekar, S. Mandavgade and W. B. Gurnule, Studies of chelating ion exchange properties of novel copolymer derived from salicylic acid and semicarbazide, J. Information, Knowledge and Research in Humanities and Social, 1(4), (2016), 177-187. [20] N. C. Das, and W. B. Gurnule , Removal of Heavy Metal Ions from Water Using Chelating Copolymer Resin-IV Derived from 2-Hydroxy, 4-Methoxybenzophenone, 1, 5-Diaminonaphthalene and Formaldehyde, International Journal of Science and Research Archive, 12(01), (2024),3058–3065. [21] W. B. Gurnule, and D. B. Patle, Preparation, characterization and chelating ion-exchange properties of terpolymer resin derived from o-aminophenol, urea and formaldehyde, Elixir Appl. Chem., 50, (2012), 10338- 10345. [22] R. N. Singru, V. A. Khati, W. B. Gurnule, A. B. Zade and J. R. Dontulwar, Studies on semiconducting, chelating and thermal properties of p-cresol-oxamide-formaldehyde terpolymer resin, Anal. Bioanal. Electrochem, 1(3), (2011), 67-86. [23] W. B. Gurnule, and S. S. Dhote, Preparation, characterization and chelating ion- exchange properties of copolymer resin derived from 2,4-dihydroxybenzoic acid ethylene diamine and formaldehyde, Der Pharma Chemica ,4 (2), (2012), 791-799. [24] S. S. Rahangdale, Synthesis and chelation Ion-exchange properties of 2,4-HABF-IV terpolymer resin,World Appl. Sci. J., 21 (2), (2013), 237-243.
Copyright
Copyright © 2024 N. C. Das, W. B. Gurnule. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63689
Publish Date : 2024-07-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

